
A D V A N C E D M A T E R I A L S & P R O C E S S E S | M A R C H 2 0 1 6
2 4
DISCOVERYOF Q-PHASES AND
DIRECT CONVERSIONOF CARBON
INTODIAMONDAND h-BN INTO c-BN
The discovery of new phases of carbon and direct conversion of carbon into
diamond and diamond-like materials
—
at ambient conditions and without a
catalyst
—
is a breakthrough with tremendous potential for electronics and
hard-materials applications.
Jagdish Narayan,* FASM, Anagh Bhaumik, and Roger Narayan,* FASM
Department of Materials Science and Engineering
North Carolina State University, Raleigh
*Member of ASM International
B
ecause graphite is the stable form
of carbon, its conversion to dia-
mond at ambient pressures and
temperatures goes against equilibrium
thermodynamics and the carbon phase
diagram. The phase diagram shows this
can be done only at very high pressures
and temperatures (>120,000 atm and
5000K), which is expensive and energy in-
tensive with limited throughput. Carbon
to diamond conversion at ambient pres-
sures and lower temperatures is scien-
tifically challenging with immense tech-
nological significance
[1-4]
. Conversion of
carbon intodiamondhas beena scientific
quest for many years. Diamond is a high-
ly desirable material with applications
ranging from abrasives, protective coat-
ings, and biomedical uses to electronics,
photonics, and display devices.
Conventional bulk processing in-
volves high pressures and tempera-
tures
[1]
, and chemical vapor deposition
(CVD) of thin films requires high tem-
peratures in the presence of hydro-
gen
[5]
, requirements that lead to low
production volumes and high costs.
Formation of nanodiamond from sil-
icon carbide (SiC) has been reported
at temperatures of ~1000
°
C under
flowing hydrogen and chlorine gases
at ambient pressure
[6]
. According to
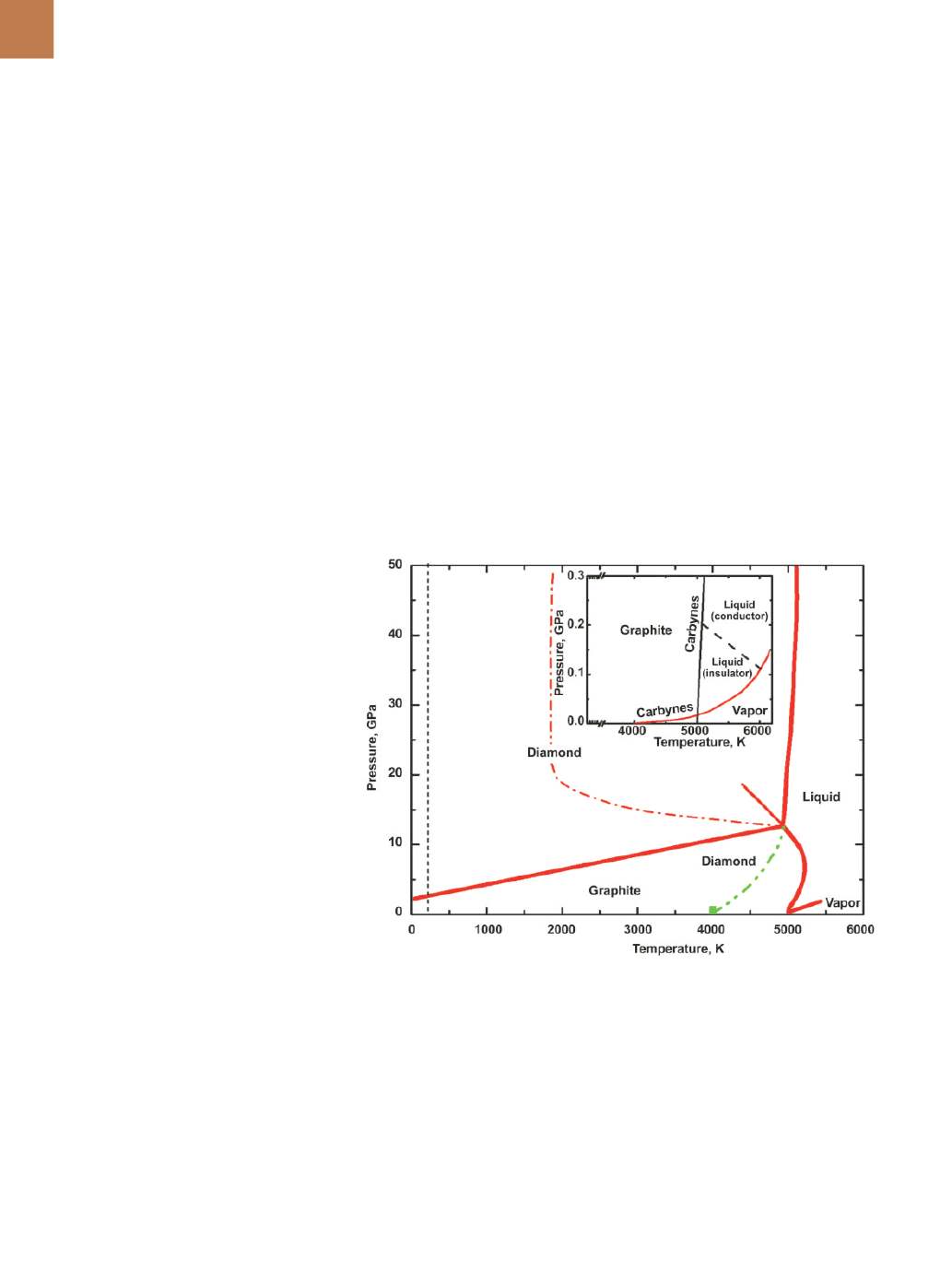
the equilibrium phase diagram (Fig. 1),
graphite, diamond, liquid, and vapor
are thermodynamically stable forms
of carbon
[1]
. At low pressures, graphite
converts directly into vapor above a
temperature of roughly 4000K. Dia-
mond synthesis from liquid carbon re-
quires even higher temperatures and
pressures, as the graphite/diamond/
liquid carbon triple point occurs at
5000K/12 GPa, where 1 GPa = 9869 atm.
Fig. 1 —
Carbon phase diagram in which amorphous diamond-like carbon melting is intro-
duced at 4000K at ambient pressures (dotted green line)
[1]
.


















