
A D V A N C E D
M A T E R I A L S
&
P R O C E S S E S |
M A R C H
2 0 1 6
2 7
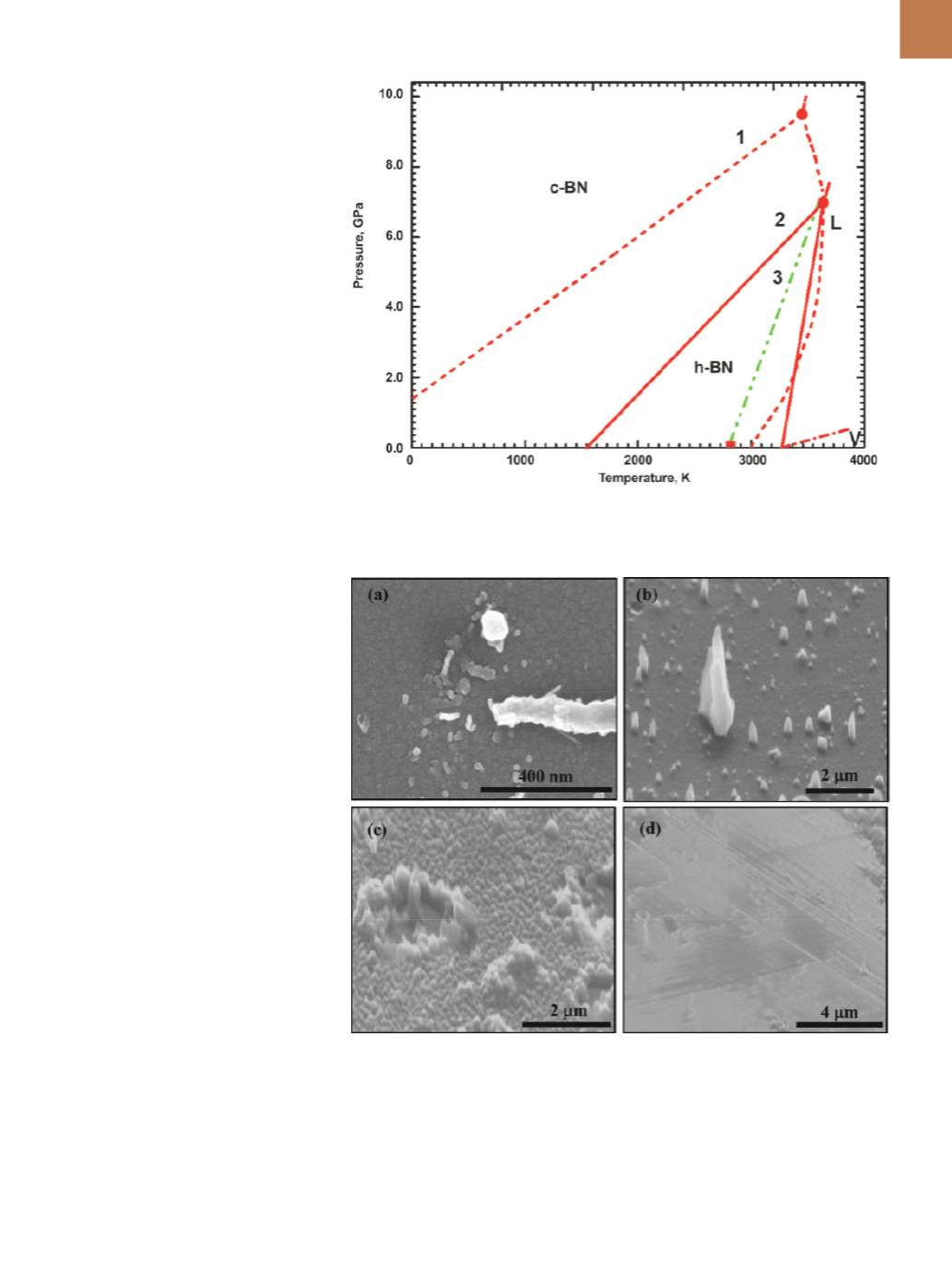
According to the phase diagram, the
transformation from h-BN into c-BN can
occur only at high temperatures and
pressures, as the hBN-cBN-liquid triple
point is at 3500K/9.5 GPa. Nanosecond
laser melting creates a super-under-
cooled state and shifts the triple point
to as low as 2800K and atmospheric
pressure. Rapid quenching from the su-
per-undercooled state leads to forma-
tion of Q-BN. The c-BN phase is nucle-
ated from Q-BN depending on the time
allowed for nucleation and growth.
Figure 4 shows the BN phase dia-
gram in the pressure and temperature
range of 0-10 GPa and 0-4000K, re-
spectively, containing regions of phase
stability for c-BN, h-BN, liquid, and va-
por
[11]
. According to the phase diagram
of Corrigan and Bundy (curve 1), the
c-BN line intersects the pressure axis at
1.4 GPa at 0K without crossing the tem-
perature axis, making h-BN the stable
phase in the entire temperature range
of 0 to 3000K, above which BN turns
into vapor
[11]
. Solozhenko, et al., tried
to refine the phase diagram based on
experimental data on BN melting and
extrapolation of specific heats of vari-
ous BN polymorphs into high-tempera-
ture regions. This modification (curve 2)
shifts the L-cBN-hBN triple point from
3500K/9.5 GPa (Corrigan-Bundy P-T dia-
gram) to 3700K/7.0 GPa, and shows cBN
is the stable phase in the temperature
range of 0 to1600K, and h-BN beyond
that at atmospheric pressure
[12-13]
.
Figure 5a shows the formation of
Q-BN and nano and microcrystallites of
c-BN after treatment with a single laser
pulse. Figure 5b shows the formation
of single-crystal nanoneedles and mi-
croneedles of c-BN; some microneedles
are over two microns long. The mech-
anism of nanoneedle and micronee-
dle formation is illustrated in Fig. 5c,
where interfacial instability in super-
undercooled BN leads to the forma-
tion of periodic features on the order of
90 nm, which coalesce to form larger
size microneedles.
Large-area single crystal thin films
are formed in the middle of the laser
beam, where there is 100% conversion
of h-BN into phase pure c-BN (Fig. 5d).
Thus, c-BN structures in the form of
nanodots, nanorods, microcrystalline
thin films, and large-area single crystal
c-BN thin films are formedby controlling
laser parameters. These structures are
phase-pure c-BN with 100% conversion
from h-BN into c-BN
[14]
. CVD methods
for c-BN processing are not well estab-
lished, and only 85% phase-pure c-BN
Fig. 4 —
BN phase diagram showing P-T phase space for stability of h-BN, c-BN, and liquid BN
(L): Dotted lines based on Bundy
[11]
, dotted lines are recent modifications
[12-13]
, and dash-dot line
extension for super-undercooling
[11]
.
Fig. 5 —
SEMmicrographs: (a) nucleation of c-BN fromQ-BN (mechanism of h-BN to c-BN
conversion), (b) formation of c-BN nanoneedles andmicroneedles up to three microns long,
(c) mechanism of initial stages of formation of nanostructures consisting of their coalescence
and nanoneedle evolution, and (d) formation of large area flat c-BN films.


















