
A D V A N C E D
M A T E R I A L S
&
P R O C E S S E S | J A N U A R Y
2 0 1 7
2 1
TENSILE PROPERTIES
Low-temperature transformation
bainite is harder than previously ach-
ieved, with values in excess of 700 HV.
Selected alloy compositions along with
transformation conditions and micro-
structural parameters of second-gen-
eration nanostructured bainitic steels
are shown in Table 1. After transfor-
mation at 220° and 250°C, hardness
values are always over 600 HV30 with
a bainitic ferrite plate thickness of
30-40 nm. Corresponding strength and
ductility data of the selected micro-
structures, in terms of yield strength
(YS), ultimate tensile strength (UTS),
uniform elongation (UE), and total elon-
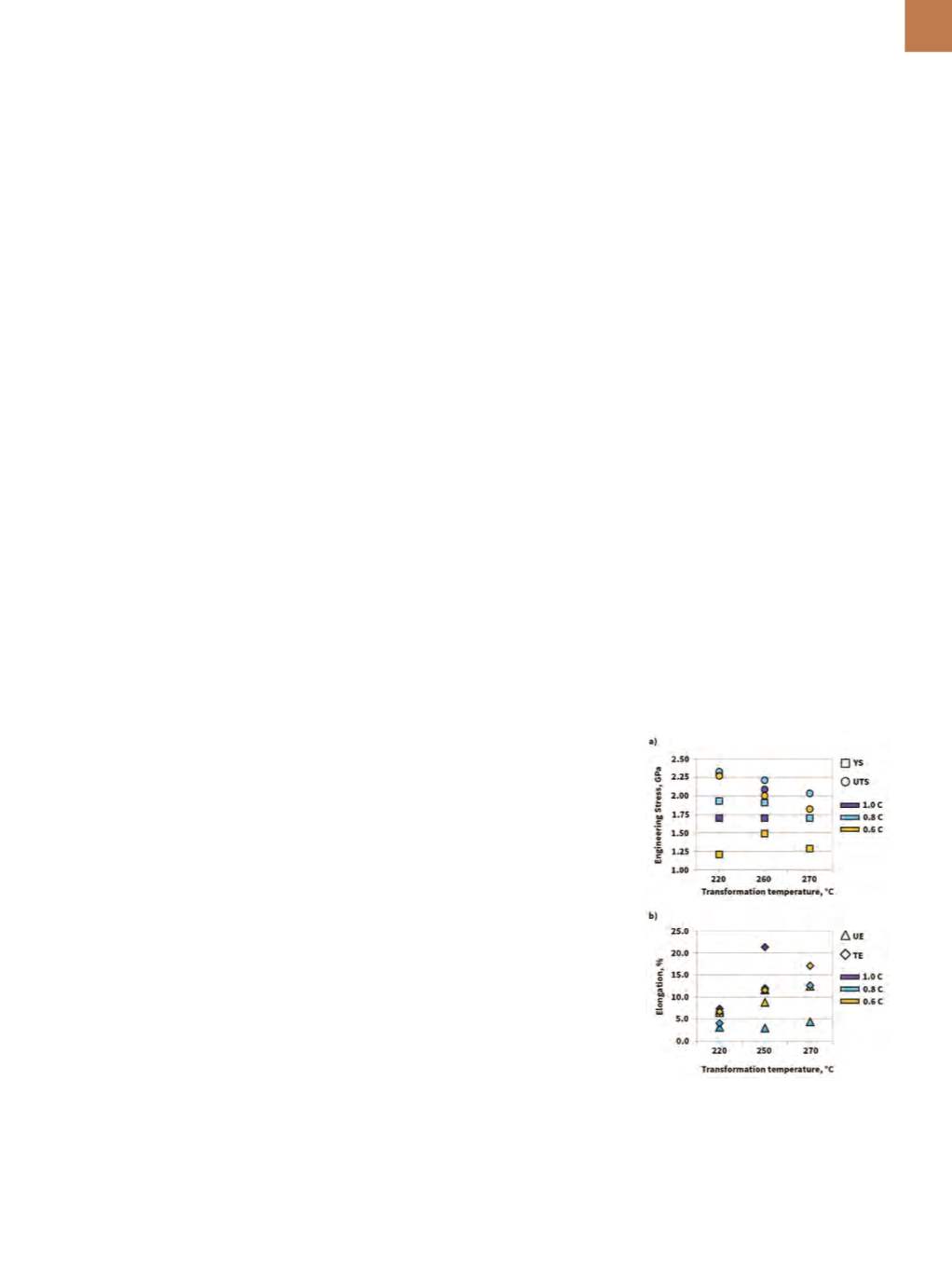
gation (TE) are illustrated in Fig. 1.
A
nanostructured
bainite
has
been developed by heat treating
high-carbon, high-silicon steels.
The new material is being produced in
bulk and affordably without using severe
deformation or complex heat treatments.
The bainitic structures consist of nano-
scale ferrite crystals 20-60 nm thick
interwoven by austenite. Nanostruc-
tured bainite has one of the highest
known densities of ferrite/austenite
interfaces. The material has the highest
strength/toughness combination ever
recorded in bainitic steels (~2.2 GPa/40
MPa·m
1/2
)
[1]
and has extraordinary roll-
ing-sliding wear performance
[2]
. This
article discusses the characteristics and
significance of nanostructured bainite in
terms of the transformationmechanism.
HEAT TREATMENT AND
TRANSFORMATION KINETICS
Generally, lowtransformation tem-
peratures lead to fine-grained micro-
structures that have both strength and
toughness. First-generation nanostruc-
tured bainitic steels were designed us-
ing models based on the atomic mech-
anism of displacive transformation
theory
[3]
. The bainite start temperature
(B
s
)—the highest temperature at which
bainite can be formed—was lowered
mainly due to high carbon concentra-
tions. In addition, the alloys contained
enough silicon to suppress cementite
precipitation from austenite. Cementite
is a splitting and void-initiating phase,
best eliminated from strong steels. The
tradeoff to achieve the nanoscale in
these bainitic steels is extremely long
transformation times. For instance,
the bainite reaction in first-generation
nanocrystalline bainitic steels took up
to 90 days at a transformation tempera-
ture of 125°C
[3]
. However, rapid heat
treatment may be required on a com-
mercial basis.
Alloy composition can be tailored
to increase the magnitude of free ener-
gy change that accompanies austenite
decomposition (ΔG
γα
= G
α
− G
γ
), where
G
α
is the Gibbs free energy of ferrite and
G
γ
is the Gibbs free energy of austen-
ite; thus accelerating both nucleation
and growth rates. Theoretical design
in second-generation nanostructured
bainite led to processing times of hours
as opposed to days by reducing carbon,
manganese, chromium, and molybde-
num contents and by refining the pri-
or austenite grain size with the help of
niobium additions
[2]
. However, the ef-
fect of the prior austenite grain size on
bainite kinetics appears to accelerate
the reaction through coarse prior aus-
tenite grains
[4]
. Coarse austenite grains
increase growth rate and decrease nu-
cleation sites of bainitic transformation.
Growth rate is more important than nu-
cleation, so the overall outcome was ac-
celeration of the bainite reaction.
The latest research into accelerat-
ing the bainite reaction suggests partial
martensite transformation with subse-
quent transformation upon up-heat-
ing
[5]
. Though the quenching and bain-
ite transformation process occurs more
quickly than in direct isothermal trans-
formation, industrial production lines
are not yet prepared for this kind of heat
treatment.
DEVELOPING NANOSTRUCTURED METAL
AT THE ATOMIC ANDNANO SCALES
Affordable bulk production of a newly developed nanostructured bainitic steel is
possible without using severe deformation or complex heat treatments.
Rosalia Rementeria, Francisca G. Caballero , Lucia Morales-Rivas, and Carlos Garcia-Mateo
Fig. 1
—
Tensile properties on select grades
and transformation conditions.


















