
A D V A N C E D
M A T E R I A L S
&
P R O C E S S E S | J U L Y / A U G U S T
2 0 1 6
2 1
For more information:
David Spen-
ciner, FASM, is a research fellow, DePuy
Synthes, Mitek Sports Medicine, 325
Paramount Dr., Raynham, MA 02767,
508.828.3721,
dspencin@its.jnj.com,
depuysynthes.com. Co-authors include
Dennis Burke and Samir Bhattacharyya.
References
1. J.L. Basko-Piluska, J.P. Thyssen, et al.,
Cutaneous and Systematic Hypersen-
sitivity Reactions to Metallic Implants,
Dermatitis,
V 22(2), p 65-79, 2011.
2. A. Weiler, R.F. Hoffmann, et al., Bio-
degradable Implants in Sports Medi-
cine: The Biological Base,
Arthroscopy,
V 16 (3), p 305-21, 2000.
3. J.M. Brady, D.E. Cutright, et al.,
Resorption Rate, Route of Elimination,
and Ultrastructure of the Implant Site
of Polylactic Acid in the Abdominal
Wall of the Rat,
J. Biomed Mater. Res.,
V 7 (2), p 155-66, 1973.
4. F.A. Barber and W.D. Dockery, Long-
Term Absorption of Poly-L-Lactide Acid
Interference Screws,
Arthroscopy,
V 22 (8), p 820-6, 2006.
5. T. Poandl, S. Trenka-Benthin, et al.,
A New Faster-Absorbing Biocomposite
Material: Long-Term In-Vivo Tissue
Reaction and Absorption, poster pre-
sentation, Spring AANA, May 2005.
6. F.A. Barber and S.A. Hrnack, Poly
L-Lactide Co-Glycolide/
β
-Tricalcium
Phosphate Interference Screw Fixation
for Bone-Patellar Bone Anterior Cruci-
ate Ligament Reconstruction,
J. Knee
Surg.,
V 26, p 423-8, 2013.
7. P. Randelli and R. Compagnoni,
et al., Long-Term Degradation of a
Poly-Lactide Co-Glycolide/
β
-Tricalcium
Phosphate Biocomposite Anchors in
Arthroscopic Bankart Repair: A Pro-
spective Study,
Arthroscopy,
V 30 (2),
p 165-71, 2014.
8. H.E. Bourke and L.J. Salmon, et al.,
Randomized Controlled Trial of Osteo-
conductive Fixation Screws for Anterior
Cruciate Ligament Reconstruction: A
Comparison of the Calaxo and Milagro
Screws,
Arthroscopy,
V 29 (1): p 74-82,
2013.
9. F.A. Barber, W.D. Dockery, et al., The
Degradation Outcome of Biocomposite
Suture Anchors made from Poly L-
Lactide Co-Glycolide and
β
-Tricalcium
Phosphate,
Arthroscopy,
V 29 (11),
p 1834-9, 2013.
completely absorbed and in most
cases, boney ingrowth follows in the
timeframe of approximately 2–3 years.
In addition, implants made of this
material do not cause adverse effects
such as cyst formation or soft-tissue
inflammatory reactions. For exam-
ple, one prospective clinical study
concluded, “Anchors made of 30%
β
-tricalcium phosphate and 70% PLGA
showed excellent biological efficacy,
without causing significant cyclic
lesions, producing gradual changes in
the MR signal that seems to become
equivalent to that of the glenoid tra-
becular bone at a mean of 29 months
after implantation.”
[7]
However, not
all biocomposite materials were as
successful, with one of these clini-
cal studies halted prematurely when
the other biocomposite material was
pulled from the market by the manu-
facturer due to “pretibial soft-tissue
swelling”
[8]
.
CONCLUSION
With a 12-year history of use in
humans, Biocryl Rapide remains state-
of-the-art in terms of orthopedic sports
medicine applications. Multiple pre-
clinical and clinical studies have shown
BR to be sufficiently strong for specific
orthopedic applications while almost
fully absorbing in an appropriately
short timeframe. Further, these studies
show that BR promotes formation of
new bone to backfill the volume that
was previously inhabited by the im-
plant.
~AM&P
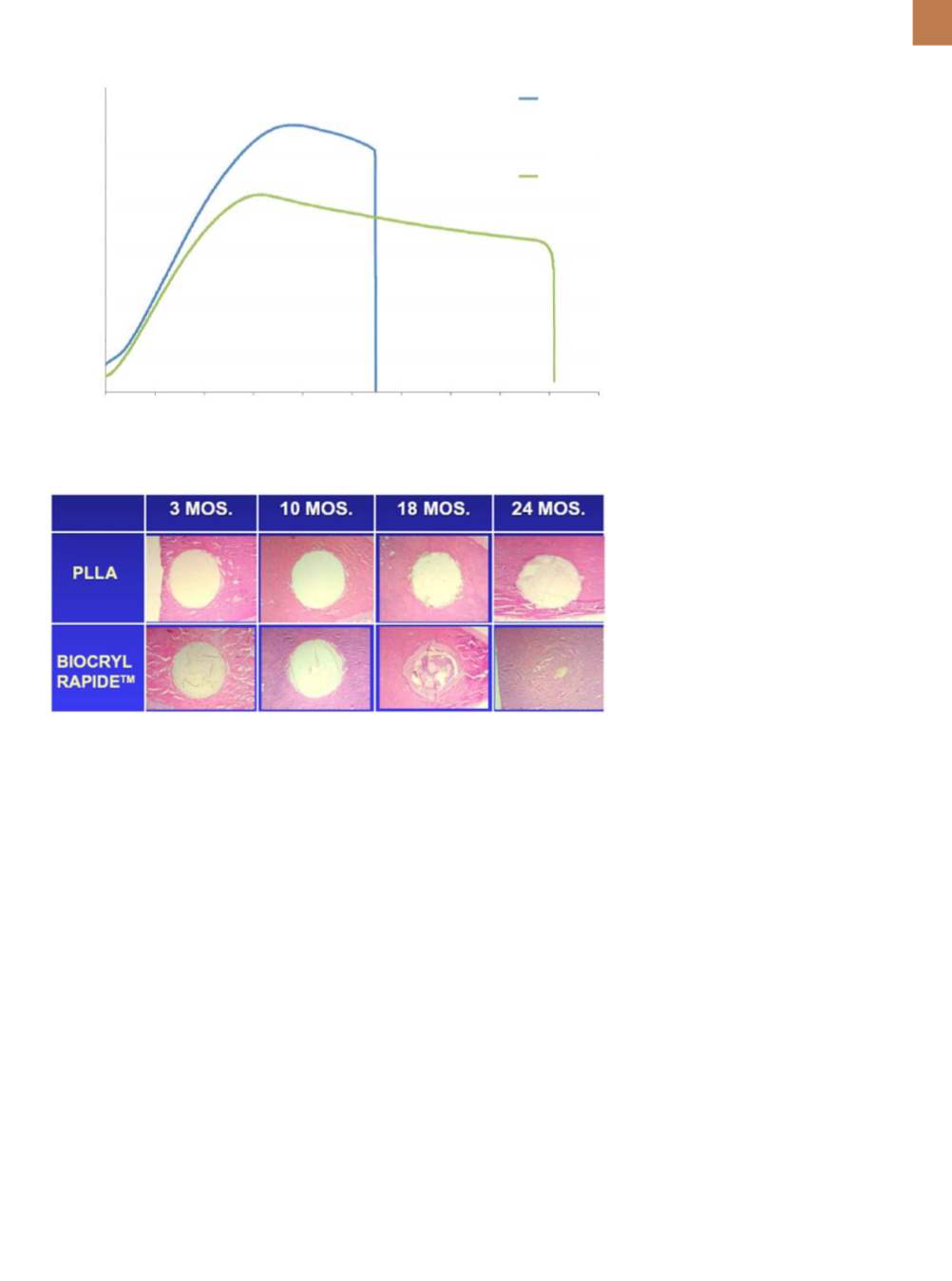
Fig. 5 —
Stress vs. strain plots for BR at room temperature (27°C) vs. body temperature (37°C).
Fig. 6 —
Histologic sections of rods (white) in bone (stained red using a hematoxylin and eosin
stain) show degradation profiles over 24 months
[5]
.
0 1 2 3 4 5 6 7 8 9 10
Strain, %
80
60
40
20
0
Stress, MPa
27
°
C
37
°
C


















