
ADVANCED MATERIALS & PROCESSES •
MAY 2014
29
However, under certain conditions experienced
during manufacturing or service, magnesium
may ignite and burn. For engineering applica-
tions, behaviors of bulk forms are of concern.
Susceptibility of magnesium and its alloys to
burning is described by the
ignition tempera-
ture,
which is defined in several different ways
throughout the literature. As an example, a
1960s study authorized by the FAA defined ig-
nition as “the point where the white flame ap-
pears and starts to propagate”
[3]
. When test
conditions are well defined, ignition tempera-
ture can be measured with reasonable repro-
ducibility. It is clear, however, that the ignition
temperature does not represent the intrinsic
parameter and in addition to differences in def-
inition, its value is affected by numerous testing
conditions. The absence of both a suitable def-
inition of ignition and well standardized meth-
ods of determining the ignition temperature of
combustible metals is the reason why literature
data are often difficult to compare.
On the other hand, the term
flammability
characterizes the susceptibility of an alloy to ig-
nite and burn after contact with a flame or an-
other heat source. Quite often, no distinction is
made between ignition and flammability and
both terms are used interchangeably in the lit-
erature. However, flammability should be seen
as a different quantity. Although the reaction of
magnesium with oxygen is exothermic in na-
ture, and releases substantial heat, ignition may
not lead to burning if sufficient heat is removed.
Alloys developed specifically for high-temper-
ature service may resist ignition or may self-ex-
tinguish if ignited.
Different measures exist for characterizing
ignition and flammability. During ignition test-
ing, an emphasis is placed on temperature,
whereas flammability testing focuses on time.
In practice, it involves a set of time periods dur-
ing contact with a flame of well-defined charac-
teristics: At first, the flame does not lead to
ignition, then ignition occurs but is self-extin-
guished after flame removal, and finally the
magnesium ignites and burns despite flame re-
moval. Both temperature and time are interde-
pendent. During ignition testing, the longer
heating time, i.e., lower heating rate, reduces
the ignition temperature. Then, during flam-
mability testing, the lower flame temperature
delays the onset of magnesium burning for a
longer period.
Suppressing surface reactivity
via alloy chemistry
The surface reactivity of magnesium is af-
fected by alloying additions, a key requirement
of structural materials. The ignition tempera-
ture of pure Mg, typically in the range of 630°-
640
o
C, is reduced by alloying elements such as
Al, Zn, Cd, Mn, and Si, which is explained
through lowering the liquidus temperature. By
contrast, rare earths and other elements with
high affinity to oxygen, together referred to as
reactive elements, show the opposite effect. Al-
though the liquidus temperature is reduced
through alloying with reactive elements, the ig-
nition temperature increases.
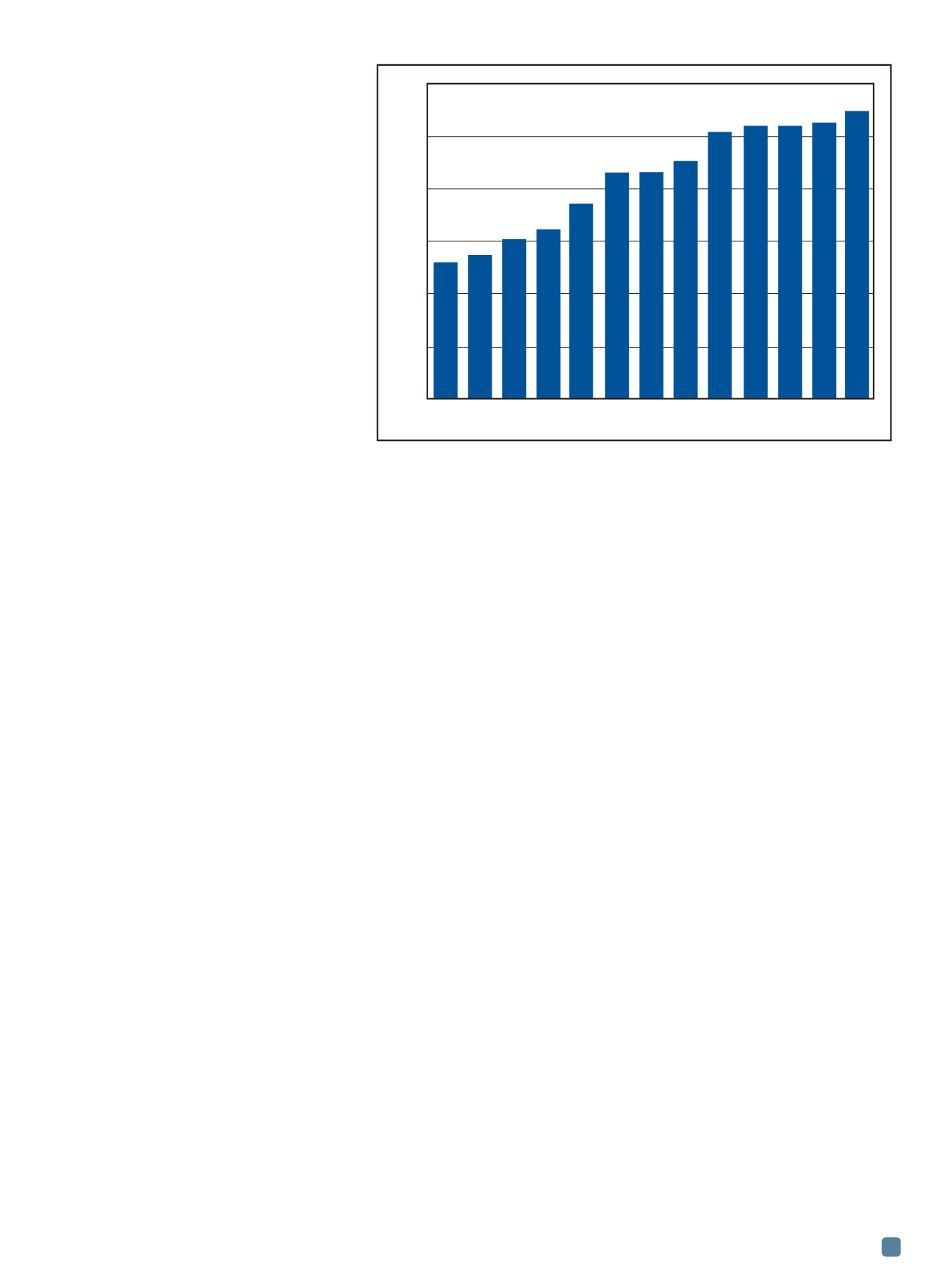
Examples of changes in the ignition tem-
perature as a result of alloying are shown in Fig.
2. A common conclusion based on the available
literature is that minor additions of rare earths
such as Y, Ce, Nd, Dy, Gd, Er, or La cause a
sharp increase in the ignition temperature of
magnesium. The minimum effective amount,
often as low as 0.1%, depends on the particular
reactive element and the base alloy chemistry.
In some cases, an optimum content exists, be-
yond which the opposite effect occurs, thereby
reducing the ignition temperature. Of reactive
elements beyond the rare earths list, Ca is
proven to be very effective in raising the igni-
tion temperature of magnesium alloys. To sup-
press ignition, Ca also may be added as a CaO
oxide dispersion, widely available.
Understanding the role of small amounts of
reactive elements is of strategic importance due
to their high cost and limited availability.
Therefore, similarity to the reactive element ef-
fect in high-temperature materials that form
chromia, alumina, or NiO protective scales
2.32 Zn 5.44 Al 1.42 Er 0.54 La 0.53 Pr 0.53 Ce 0.56 Sr 1.74 Si 0.48 Gd 0.50 Sm 0.55 Y 1.46 Dy 1.22 Ca
Mg-X binary alloys
800
700
600
500
400
300
200
Ignition temperature (°C)
Fig. 2 —
Ignition temperature of binary Mg alloys tested during continuous heating.
Based on data from
[9]
.


















