
A D V A N C E D M A T E R I A L S & P R O C E S S E S | A P R I L 2 0 1 5
1 6
NANOTECHNOLOGY
NEW FORMULA FOR
IDENTIFYING SUITABLE
GRAPHENE SUBSTRATE
Physicists fromForschungszentrum
Jülich, Germany, developed criteria with
which scientists can seek suitable sub-
strate materials for graphene in a target-
ed way. Interactions with the substrate
material often remove the amazing
properties that characterize this special
form of carbon. Together with partners
at other institutions, scientists demon-
strated that the influence exerted by the
substrate on the electronic properties of
graphene can be estimated by means of
a simple structural parameter.
Harder than diamond, tougher than
steel, and many times more conductive
than silicon—these and further extraor-
dinary properties are the reason why
graphene is intensively studied world-
wide. Thematerial is only one atomic lay-
er thick. Its use, however, is so far mostly
limited to laboratory experiments. One
of the major tasks on the way to practi-
cal applications is the search for suitable
substrate materials without which the
extremely thin material is of little use.
“We simply wanted to find an ac-
cessible parameter which can be used
to compare different substrates di-
rectly,” reports François Bocquet. “The
decisive criterion turned out to be the
atomic distance between the graphene
layer and the underlying substrate,” he
explains.
For more information: François
Bocquet, +49 2461 61-3987,
f.bocquet@ fz-juelich.de,
www.fz-juelich.de.
BRINGING CLEAN ENERGY
A STEP CLOSER
Researchers at Case Western Re-
serve University, Cleveland, have shown
that an inexpensive metal-free catalyst
performs as well as costlymetal catalysts
at speeding the oxygen reduction reac-
tion in an acidic fuel cell for the first time.
The carbon-based catalyst also corrodes
less than metal-based materials and has
proved more durable. The findings are
major steps toward making low-cost
catalysts commercially available, which
could, in turn, reduce the cost to gener-
ate clean energy fromPEM fuel cells—the
most common cell being tested and used
in cars and stationary power plants.
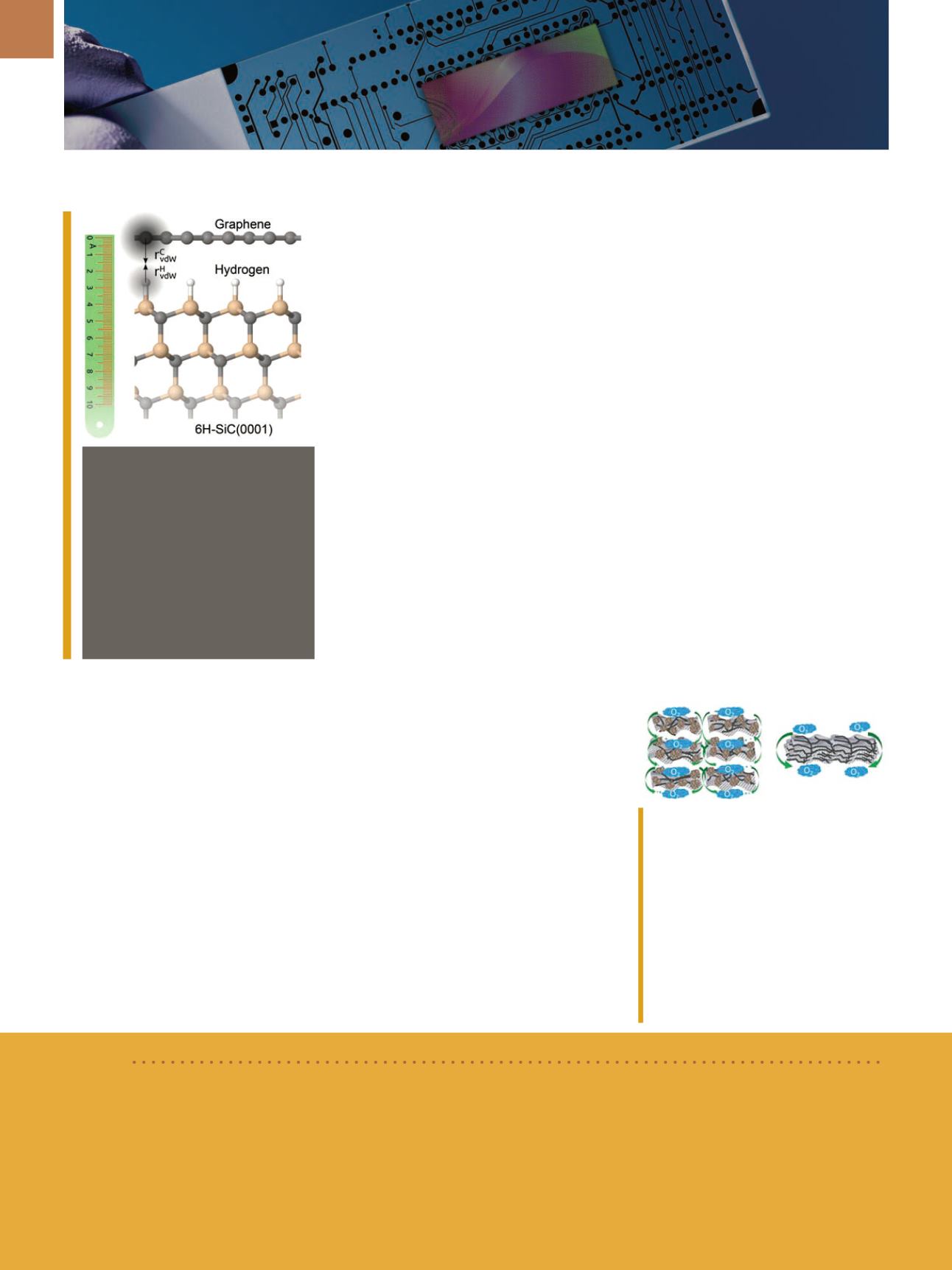
Graphene on a silicon carbide
substrate whose surface was
treated with hydrogen in order to
electrically decouple the graphene.
The distance between the two lay-
ers, minus the respective van der
Waals radii, gives an approximate
value for the interaction strength.
Courtesy of Sforzini et al.,
Physical
Review Letters
/The American Physi-
cal Society.
The key to the new catalyst is its ra-
tionally designed porous structure, says
Liming Dai, the Kent Hale Smith Pro-
fessor of macromolecular science and
engineering at Case. Researchers mixed
sheets of nitrogen-doped graphene, a
single-atom thick, with carbon nano-
tubes and carbon black particles in a
solution, then freeze-dried them into
composite sheets and hardened them.
Graphene provides enormous surface
area to speed chemical reactions, nano-
tubes enhance conductivity, and carbon
black separates the graphene sheets for
free flow of the electrolyte and oxygen,
which greatly increased performance
and efficiency. Researchers found that
those advantages were lost when they
allowed composite sheets to arrange
themselves in tight stacks with little
room between layers.
For more infor-
mation: Liming Dai, 216.368.4176,
liming. dai@case.edu,
www.case.edu.
BRIEF
The president’s budget for fiscal year 2016 provides $1.5 billion for the
National Nanotechnology Initiative
(NNI), a continued
Federal investment in support of the president’s priorities and innovation strategy. Cumulatively totaling more than $22 billion
since the inception of the NNI in 2001, this funding reflects nanotechnology’s potential to significantly improve our fundamental
understanding and control of matter at the nanoscale and to translate that knowledge into solutions for critical national needs.
nano.gov/2016BudgetSupplempplement.
Structure enables a carbon-based
catalyst to perform comparably with
metal catalysts in an acidic fuel cell.
Carbon black agglomerates maintain a
clear distance between graphene sheets
imbedded with carbon nanotubes,
allowing oxygen and electrolyte to flow
through and speeding the oxygen-
reduction reaction (a). Without the
agglomerates, the sheets stack closely,
stalling the reaction (b).
(a) (b)


















