
news
industry
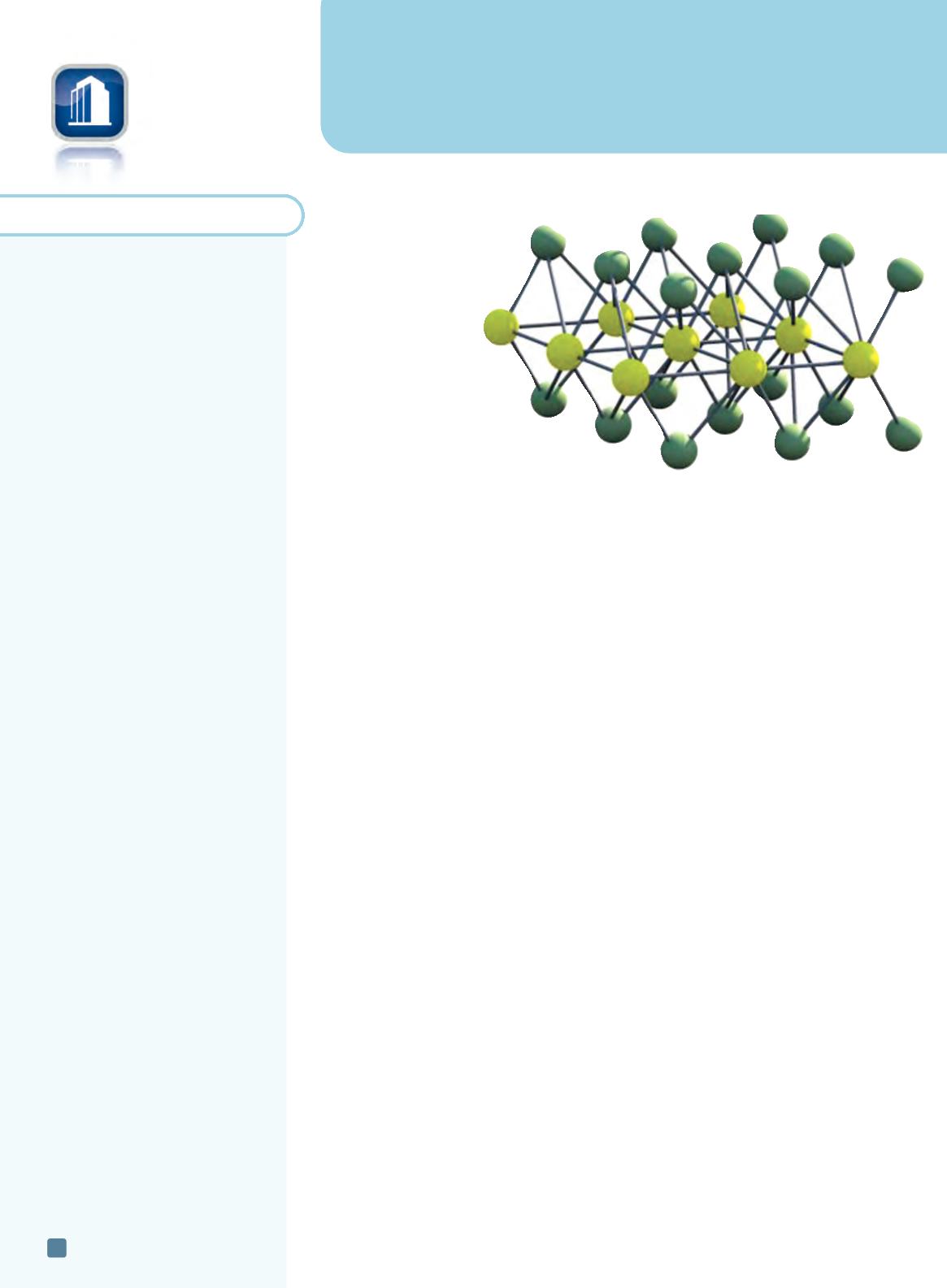
Making the thinnest solar cells
Researchers at Vienna Uni-
versity of Technology, Austria,
created a diode made of tung-
sten diselenide and experi-
ments show it could be
used to create ultrathin
flexible solar cells. Al-
though graphene is
often considered a wonder
material, it is not well suited
for building solar cells. “The
electronic states in graphene are
not practical for creating photovoltaics,”
says research leader Thomas Mueller. So he and his
team looked for other materials that can be arranged
in ultrathin layers but have better electronic properties.
They used tungsten diselenide, which consists of one layer of tungsten atoms connected
by selenium atoms above and below the tungsten plane. The material absorbs light, much
like graphene, but can be used to create electricity. The layer is so thin that 95% of the light
just passes through—but a tenth of the remaining 5%, which is absorbed by the material,
is converted into electrical power. A larger portion of the incident light can be used if sev-
eral of the ultrathin layers are stacked on top of each other—but sometimes high trans-
parency is a useful side effect. “We envision solar cell layers on glass facades, which let part
of the light into the building while at the same time creating electricity,” says Mueller.
For
more information: Thomas Mueller,
thomas.mueller@tuwien.ac.at, 43 1/58801-38739,
www.graphenelabs.at.
Discovery could lead to better electric vehicle batteries
An international research team led by Western University, Ontario, investigated elec-
tric batteries and battery materials to reveal an underlying mechanical interaction that oc-
curs during the carbon coating process. The coating not only affects the conductivity and
performance of battery materials, but also alters the chemistry of the battery material’s in-
teractive surface. Using advanced measuring techniques, including scanning electron mi-
croscope (SEM) imaging, researchers discovered that the surface of the LiFePO
4
battery
materials may actually melt during the heating process (at 600°-900°C), and that this phase
change is size-dependent.
“By carbon coating at a relatively high temperature, the surface of LiFePO
4
battery ma-
terials basically becomes a liquid, creating island-shaped phases or pockets on the top of
the battery materials, which breaks its conductivity,” explains Xueliang (Andy) Sun. He also
notes the discovery has yet to solve the problem of building better electric car batteries, but
understanding the surface chemistry greatly enhances the possibility to do so.
For more
information: Xueliang (Andy) Sun, 519/661-2111 ext. 87759,
xsun@eng.uwo.ca,
www.eng.uwo.ca.
Californium could be used to safely store radioactive waste
Californium is “wicked stuff,” according to Florida State University, Tallahassee,
professor Thomas Albrecht-Schmitt. In carefully choreographed experiments, re-
searchers found that californium had amazing abilities to bond and separate other
materials. It was also found to be extremely resistant to radiation damage. The discov-
eries could help scientists build new storage containers for radioactive waste, plus
help separate radioactive fuel, which means the fuel could be recycled.
For more in-
formation: Thomas Albrecht-Schmitt, 850/645-0477,
albrecht-schmitt@chem.fsu.edu,
www.fsu.edu.
ADVANCED MATERIALS & PROCESSES •
MAY 2014
16
E
NERGY
T
RENDS
briefs
ASTM International,
West
Conshohocken, Pa., introduced a
new standard—ASTM E2956 –
14—Standard Guide for Monitoring
the Neutron Exposure of LWR
Reactor Pressure Vessels. The USA
Code of Federal Regulations
requires a surveillance program for
all operating LWRs to monitor
changes in the fracture toughness
properties of ferritic materials in the
reactor vessel beltline, which result
from exposure to neutron irradiation
and the thermal environment. This
data is then used to determine the
appropriate safety conditions used
throughout the vessel’s life.
www.astm.org.
Researchers from
North Carolina
State University,
Raleigh,
developed a new processing
technique that makes LEDs brighter
and more resilient by coating the
semiconductor material gallium
nitride (GaN) with a layer of
phosphorus-derived acid.
Researchers started with polar GaN,
composed of alternating layers of
gallium and nitrogen. To increase
luminescence, the material’s
surface was etched with phosphoric
acid. Phosphonic groups that self-
assemble into a monolayer on the
surface were also added. This layer
further increases luminescence and
improves the stability of GaN by
making it less likely to react
chemically with its environment.
www.ncsu.edu.
A key patent from
3M,
St. Paul,
Minn., for lithium-ion battery nickel-
manganese-cobalt (NMC) cathode
technology emerged from
reexamination at the
U.S. Patent
and Trademark Office (USPTO)
with all original claims being
confirmed as patentable and with
no amendments (U.S. Patent
7,078,128). NMC cathode technology
is widely used in lithium-ion
batteries for consumer electronics
and electric vehicles. The patented
technology enables lithium-ion
battery makers to design electrodes
for specific applications for
optimum balance of power, energy,
stability, and cost.
www.3M.com.
Tungsten diselenide.
Courtesy of TU Vienna.


















